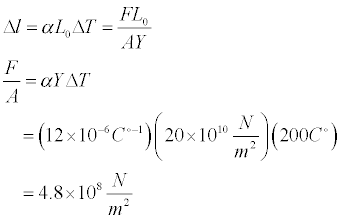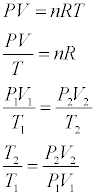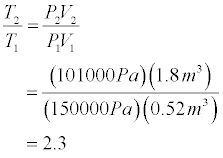Physics 151: Computerized Recitation #13
Matter under Pressure
This recitation will focus on the ideal gas law and thermal stress -- both describe the behavior of matter under changing pressure. Please experiment with the Physlet simulations below and complete the required calculations on your worksheet.
Simulation 1: Thermal Expansion and Stress
The physlet above displays a rod that is 20 m in length at -100°C. The temperature is then raised to 100°C. The resulting change in length and the thermal stress assuming this change in length was prevented are then given. Note that thermal expansion may not be visible on the scale of our animation.
| Substance | Coefficient of Thermal Expansion a | Young's Modulus Y |
| Steel | 12 x 10-6 C°-1 | 20 x 1010 N/m2 |
| Lead | 29 x 10-6 C°-1 | 1.6 x 1010 N/m2 |
|
Suggestions for Exploration
- Set up the simulation for Steel. We can solve for the thermal expansion of the steel rod with the following equation:

- We can solve for the amount of thermal stress of this expansion were not able to occur by noting that the change in length occurs in both the formula for linear expansion and the formula for Young's Modulus.
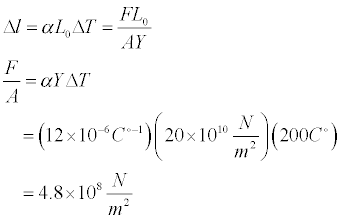
It is interesting that the change in length is so small that it is not visible at the resolution of the simulation yet the stress associated with this expansion is so large.
- Repeat the simulation for Lead and verify the output values.
Worksheet: Can you determine the coefficient of linear expanion and Young's Modulus for Kryptonite?
Simulation 2: Ideal Gas Law
A giant spherical air bubble rises in a large body of water as shown in the animation (position is in meters and time is in seconds) like what you might get from underwater industrial vents. You may control the original depth and size of the bubble. What does the ideal gas law say about how temperature changes between the initial depth and the surface?
Suggestions for Exploration
- Set up the simulation for an initial bubble radius of 0.5 m and a depth of 5.0 m. We assume in our simulation that the radius of the bubble
grows by 0.5 m/s (which is probably not very realistic). The amount of gas present in the bubble does not change between the intial depth and the surface.
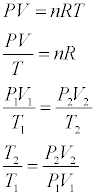
- We can now solve for the temperature ratio of the final and intial states.
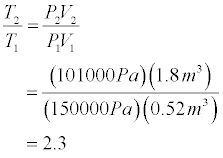
- Experiment with the other bubbles until you can predict how a parameter change will affect the simulation and the outputs.
Worksheet: Determine the values of the unknown bubble radius, unknown starting bubble depth, and the temperature ratio for these two unknown values.